Our Mission
At Nextgen Healthcare Solutions, we are committed to raising the bar on the current state of consumer diagnostics. With 50+ years of combined experience in the healthcare sector, our goal is to implement advanced diagnostic services to meet the modern requirements of an expanding healthcare market.
Our Team
Nextgen Healthcare solutions is committed to the field of healthcare innovation. Our cutting-edge formula involves the implementation of the most modern diagnostic instruments and smartest information system foundations. Our team consists of individual leaders in healthcare, diagnostics, artificial intelligence and machine learning. Our combined strengths allow us to lead the world in advancing healthcare initiatives and implementing next generation systems.
Nextgen Healthcare Solutions has extensive experience working with the entire healthcare compendium in the public and private sectors. We have implemented advanced diagnostic systems in governmental entities, as well as private hospitals.
Sienna COVID-19 Antigen Test
This rapid chromatographic immunoassay was created for the qualitative detection of COVID-19 antigen. The identification is based on the monoclonal antibodies specific for the nucleocapsid protein of SARS-CoV-2.
It is intended to aid in the rapid diagnosis of COVID-19 infection.
The test is intended for professional in vitro diagnostic use.
- FDA EUA Registered
- 98% Specificity
- Over 96% accuracy
Learn More
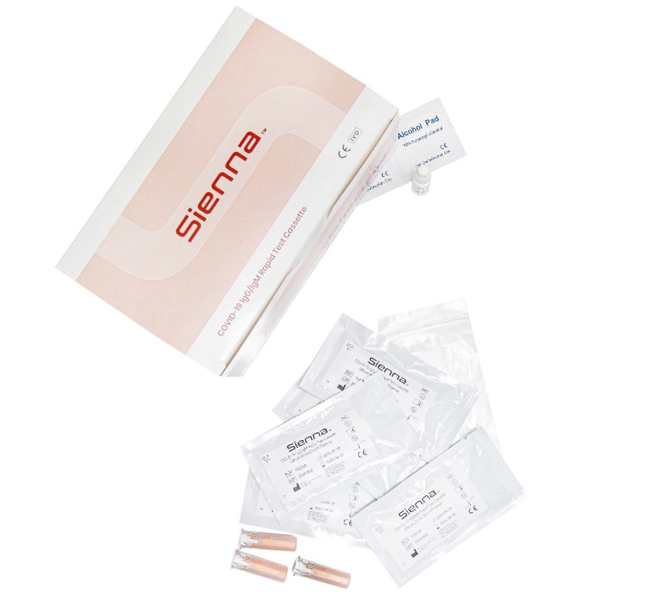
Sienna COVID-19 IgM- IgG Antibody Test
This rapid chromatographic immunoassay was created for the qualitative detection of COVID-19 antibody. The identification is based on the monoclonal antibodies specific for the nucleocapsid protein of SARS-CoV-2.
It is intended to aid in the rapid diagnosis of COVID-19 infection.
The test is intended for professional in vitro diagnostic use.
- FDA EUA Registered
- 98% Specificity
- Over 96% accuracy
Learn More
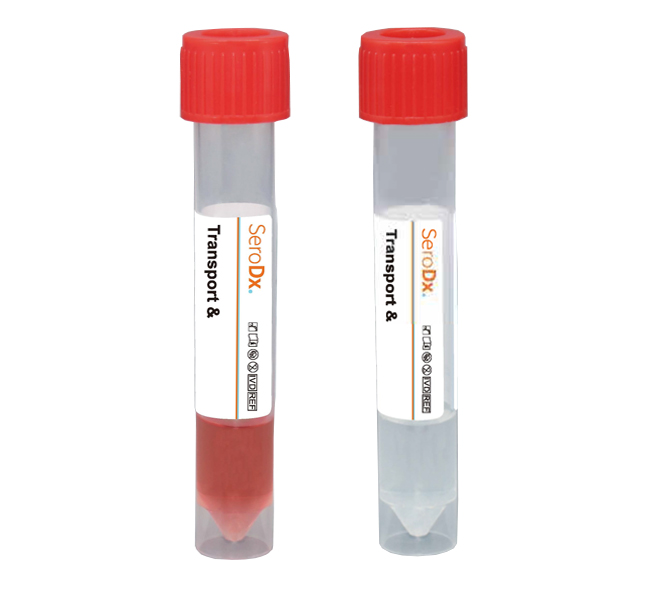
Viral Transport Medium
To provide a standard operating procedure (SOP) for producing viral transport medium (VTM) for specimens for viral culture or other means of viral detection. This SOP provides an alternative to commercially available products. There are many acceptable formulations of this medium that may be suitable for the unique conditions of individual laboratories. The specific needs of the shipping and receiving laboratories should be considered when choosing a VTM formulation.
- FDA Registered Product
Learn More